The Head of Mucosal Infection and Immunity at Imperial College Professor Robin Shattock explained their coronavirus vaccine may be. Vaccine factories are not like normal factories explains Mouser.
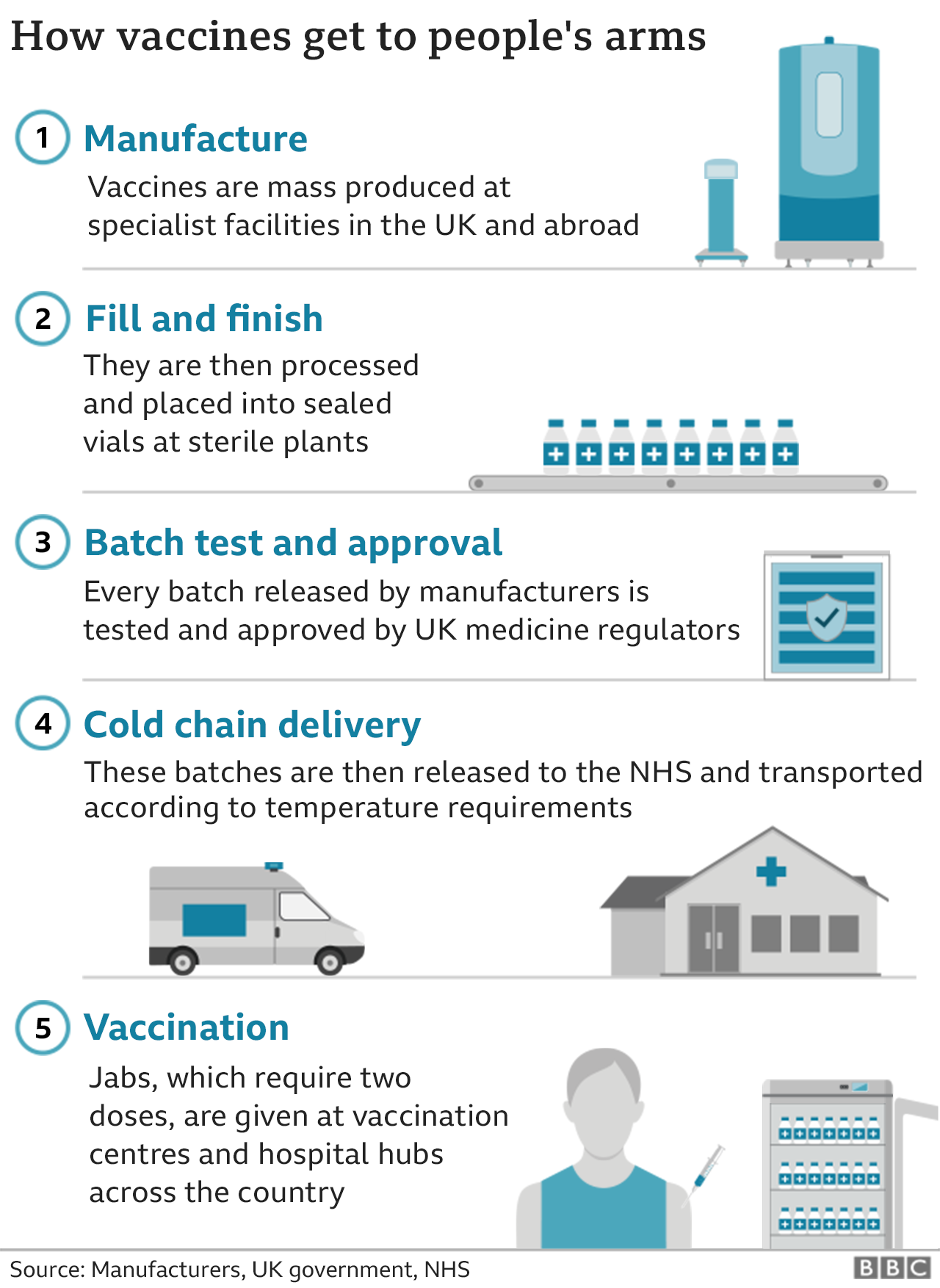 Covid Vaccine How Many People In The Uk Have Been Vaccinated So Far Bbc News
Covid Vaccine How Many People In The Uk Have Been Vaccinated So Far Bbc News
AstraZeneca the official partner for the coronavirus vaccine candidate fro Oxford for the production.

How are vaccines mass produced. World Health Organisation WHO will assist pharmaceutical companies in low and middle income countries LMICs to mass manufacture mRNA COVID-19 vaccines. They have to be mixed together precisely as nanoparticles that are not much bigger than molecules. We need to continually adapt production process to satisfy evolving regulatory demand which varies country by country.
At the facility a sterile production line could be mass producing a vaccine within weeks. How is a vaccine created. Vaccines manufacturing is a biological process where a very high level of expertise is required.
Coronavirus vaccines are begining to be rolled out with two COVID-19 shots having secured emergency authorizations from US regulators. The fluid containing virus is harvested from the eggs. Many people breathed a sigh.
Vaccines are only approved if the studies have proven to be successful safe and ethical. The second newer technique producing mRNA vaccines such as the Pfizer and Moderna jabs involves a modified spike protein being cultured in the lab and then combined with enzymes and nucleotides. Most vaccines are based in biological processes.
A vaccine typically contains an agent that resembles a disease-causing microorganism and is often made from weakened or killed forms of the microbe its toxins or one of its surface proteins. For inactivated influenza vaccines ie flu shots the vaccine viruses are then inactivated killed and the virus antigen is purified. On January 27 Health Minister Saeed Namaki said that there are four different ways to supply the coronavirus vaccine including direct purchase from a foreign country procurement from the World Health Organizations COVAX facility a joint production with a Cuban company as well as domestic production of the vaccine.
A key challenge will be mass production. If it passes all of those phases the product needs to be approved by regulators and produced. Without approval the government will not allow the vaccine to be mass-produced and commercialized.
The production of a vaccine can take between 6 and 36 months. They could start filling vials with a. Coronavirus vaccine can be mass produced explains professor.
In this case the vaccines will be shipped from the manufacturing facilities in the Northeast US. The manufacturing process continues with quality testing filling and distribution. And Europe to a distribution center in Irving Texas which will be equipped with freezers to store.
The vaccine must be approved from regulators. Once the vaccine is produced the fill-finish operation which is the process of filling vials and packaging the vaccine for distribution is handled by Catalent CTLT -04 in the US and by. While a number of vaccines are already available the road to mass production is long and bumpy.
Once the approval is given the vaccine is mass-produced. Manufacturing takes place in specialist facilities and the cost overall can be between 200 million and 500 million to do so. The small scale of production.
Not enough care with. The seemingly simple step of combining these two ingredients is seen as a major bottleneck in our current vaccine production. A vaccine is a biological preparation that provides active acquired immunity to a particular infectious disease.
Producing a vaccine is quite a challenge and requires a lot of stamina. Right now theyre training staff by filling glass vials with water. As the cases of the novel coronavirus infection rise around the world with the UK being one of the worst affected a British Pharmaceutical Company has announced that they will be undertaking mass production of the coronavirus vaccine candidate from Oxford University.
So they are made from an organism like a bacteria or a yeast or a virus that can yield a particle or protein of interest that will serve as the active ingredient in the vaccine.
 Understanding The Complexity Of Vaccine Manufacturing Sanofi
Understanding The Complexity Of Vaccine Manufacturing Sanofi
 Advances And Challenges In Vaccine Development And Manufacture Bioprocess Internationalbioprocess International
Advances And Challenges In Vaccine Development And Manufacture Bioprocess Internationalbioprocess International
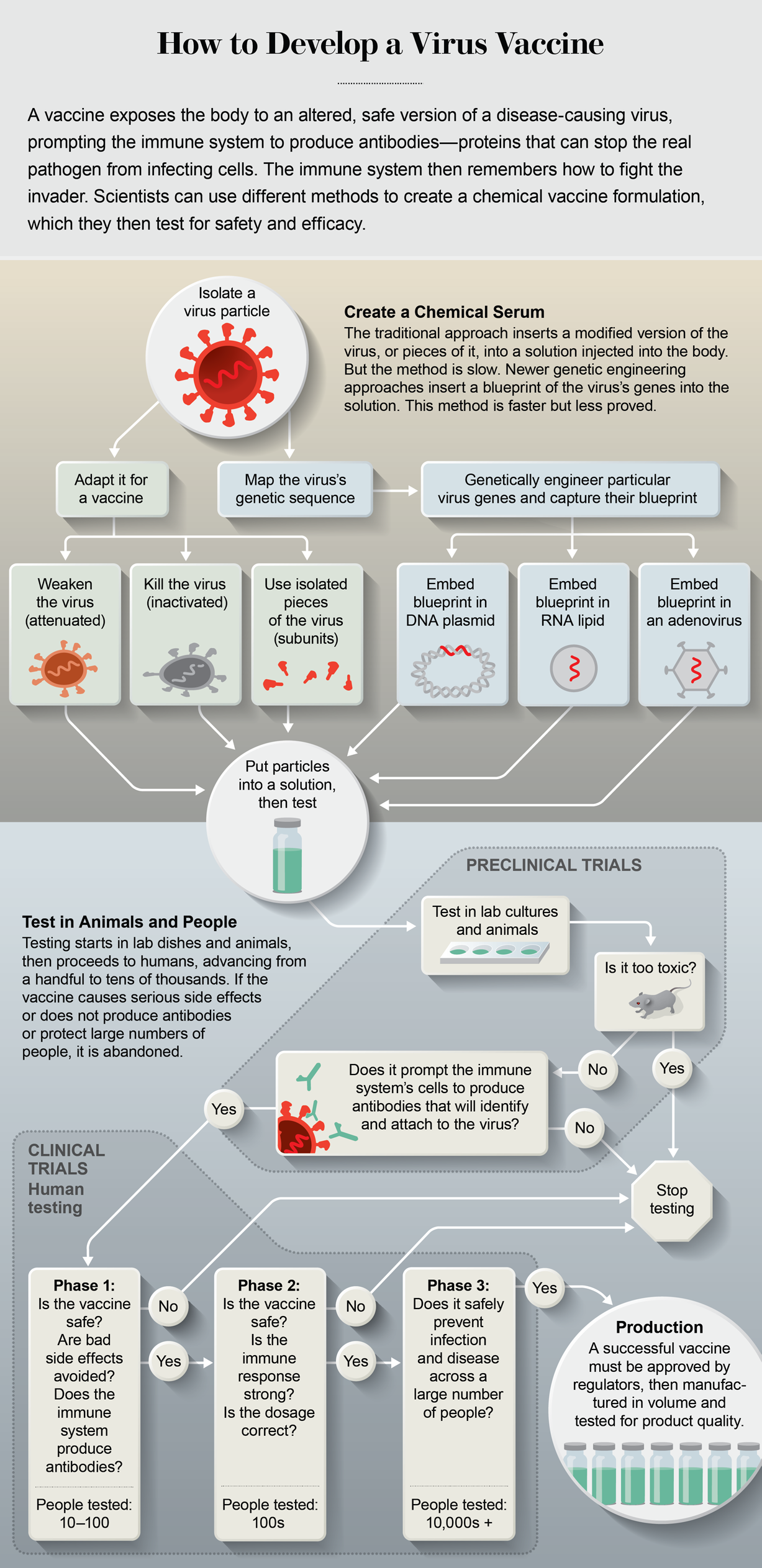 Genetic Engineering Could Make A Covid 19 Vaccine In Months Rather Than Years Scientific American
Genetic Engineering Could Make A Covid 19 Vaccine In Months Rather Than Years Scientific American
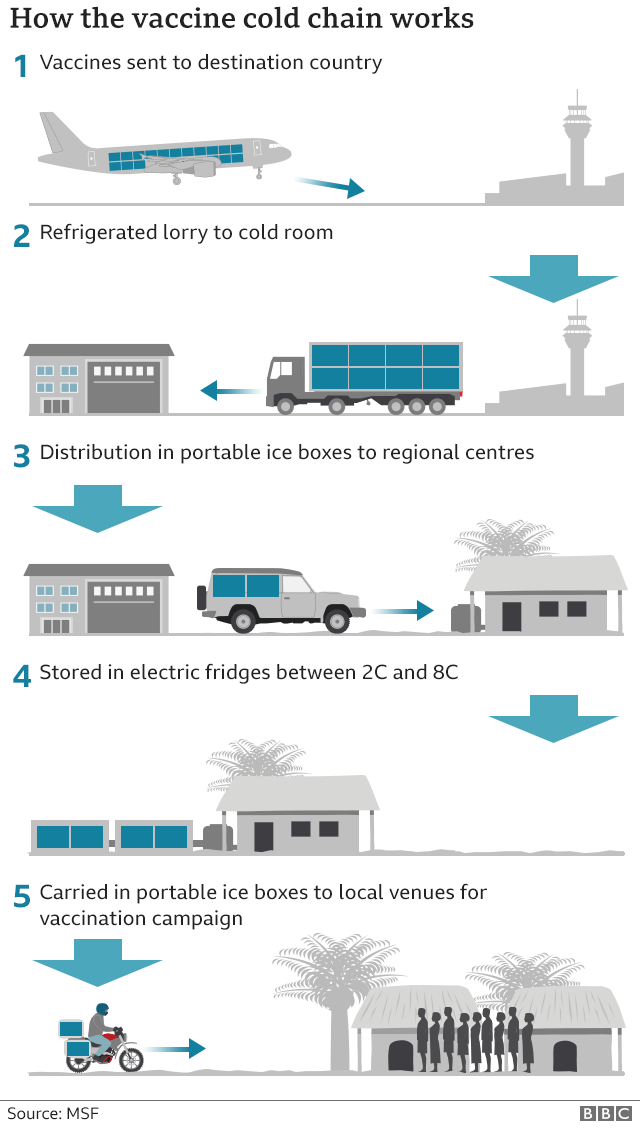 Coronavirus How Soon Can We Expect A Working Vaccine Bbc News
Coronavirus How Soon Can We Expect A Working Vaccine Bbc News
 Covid 19 Do We Need A New Vaccine Science In Depth Reporting On Science And Technology Dw 11 02 2021
Covid 19 Do We Need A New Vaccine Science In Depth Reporting On Science And Technology Dw 11 02 2021
 Advances And Challenges In Vaccine Development And Manufacture Bioprocess Internationalbioprocess International
Advances And Challenges In Vaccine Development And Manufacture Bioprocess Internationalbioprocess International
 Can The World Find A Good Covid 19 Vaccine Quickly Enough The Economist
Can The World Find A Good Covid 19 Vaccine Quickly Enough The Economist
 The Coronavirus Vaccines Will Likely Work Making Them Fast Will Be Hard The New York Times
The Coronavirus Vaccines Will Likely Work Making Them Fast Will Be Hard The New York Times
 The Big Barriers To Global Vaccination Patent Rights National Self Interest And The Wealth Gap
The Big Barriers To Global Vaccination Patent Rights National Self Interest And The Wealth Gap
 How Are Vaccines Produced Vaccines Europe
How Are Vaccines Produced Vaccines Europe
 Covid 19 Update Here S Where We Stand Now In The Race For A Vaccine Tif
Covid 19 Update Here S Where We Stand Now In The Race For A Vaccine Tif
 Manufacturing Vaccines Is A Complex Journey Sanofi
Manufacturing Vaccines Is A Complex Journey Sanofi
 Manufacturing Pace Is Key For Covid 19 Vaccine Candidates Hoping To Jump To Warp Speed Pink Sheet
Manufacturing Pace Is Key For Covid 19 Vaccine Candidates Hoping To Jump To Warp Speed Pink Sheet


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.