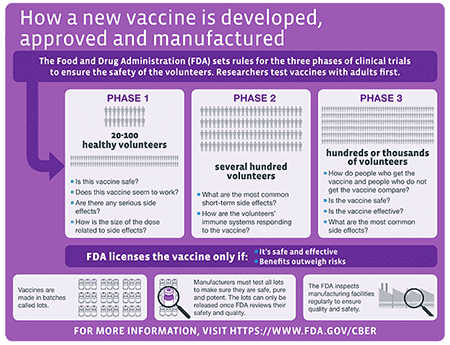
Every Covid-19 Vaccine Question Youll Ever Have Answered. Following medical case review it has been determined that 14 of these deaths are not linked to a COVID-19 vaccine and the other 12 are still under investigation.
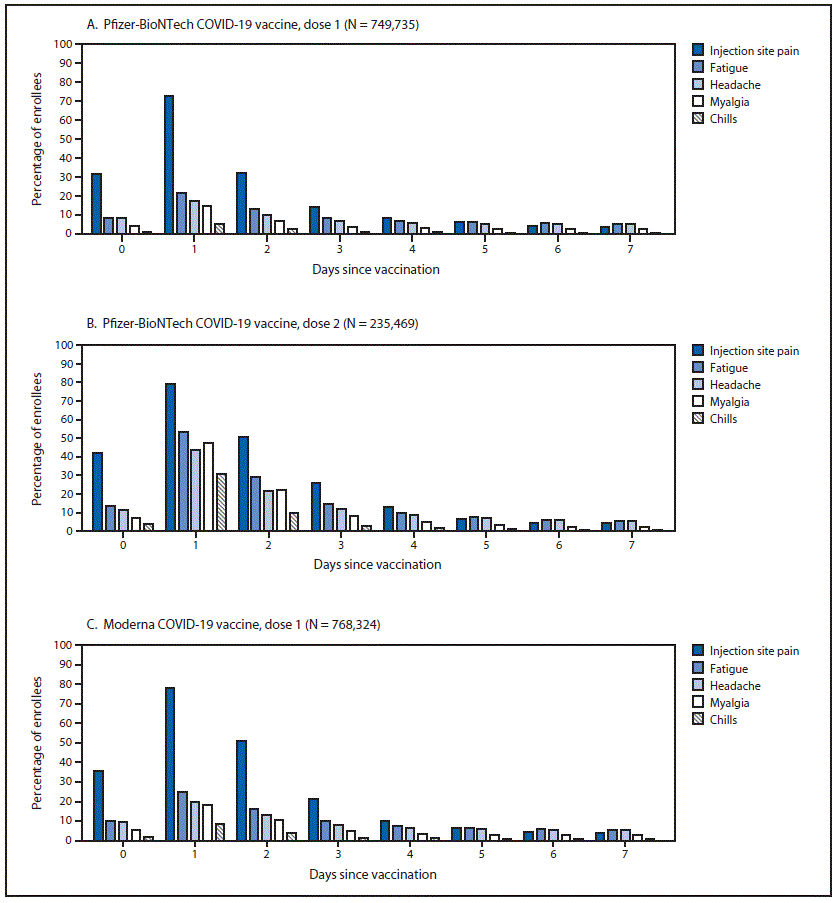 First Month Of Covid 19 Vaccine Safety Monitoring United States December 14 2020 January 13 2021 Mmwr
First Month Of Covid 19 Vaccine Safety Monitoring United States December 14 2020 January 13 2021 Mmwr
In each country 4060 of all the registered deaths are in older frail people living in.

Vaccine death rate. Compared to other years mortality is 40 times higher. It also showed males accounted for 55 percent of deaths to 43 percent of females. 15 429 were women.
The uncovering of the vaccination data in Israel reveals a frightening picture. Said individuals were given the AstraZeneca vaccine which is administered only to people. The US Centers for Disease Control and Prevention CDC estimated that during the 2018-19 season the vaccine prevented 3500 influenza-associated deaths here.
Deaths following COVID-19 vaccination are because of the vaccine As the US. Hungary is suffering a devastating surge in COVID-19 deaths despite the fact it has the highest vaccination rate in the European Union. On February 11 a Ynet article presented data related to vaccination.
Six participants did die during the 44000 person Pfizer vaccine trial two of whom were given the vaccine while the other four people received a placebo. Compared to other years mortality is. In January 2021 there were 3000 records of vaccine adverse events including 2900 for mRNA vaccines.
It said the deaths had occurred between 0 and 49 days after vaccination with 94 unknown. It set a new daily death record on Wednesday with 302. CDC and FDA physicians review each case report of death as soon as notified and CDC requests medical records to further assess reports.
16 457 after the Pfizer-BioNTech vaccine and 19 543 after the Moderna vaccine. The median interval from vaccination to death was 3 days range 020 days. Up to and including March 26 2021 a total of 26 reports identified deaths that occurred after the administration of a vaccine.
57 This means that even at the current rates of efficacy 53 of all deaths in children under-5 from rotavirus in 2016 could have been avoided by full vaccine coverage. Its worth noting for context that the deaths of over 307000 Americans have been attributed definitively to Covid-19. During this time VAERS received 3005 reports of death 000158 among people who received a COVID-19 vaccine.
In January 2021 there were 3000 records of vaccine adverse events including 2900 for mRNA vaccines. Included among the 518 76 serious reports were 35 reports of death. These 71 deaths in Europe so far must be compared with the few million who have been vaccinated.
Reaches an unfortunate landmark in coronavirus deaths over 500000 on Tuesday a number unsurpassed by any. Decedents ranged in age from 25 to 91 years median 62 years. Rates were highest in the group aged 85 years and increased from the 0-1-day to the 0-60-day interval following vaccination.
However as the chart shows full vaccine use that is a 100 coverage globally could have prevented an additional 83200 deaths. Among 13033274 vaccinated people 15455 deaths occurred between 0 and 60 days following vaccination. None of the deaths were deemed related to the vaccine treatment.
Head explains that at time of. The latest reports from March 12 indicate a total of 15 individuals died after receiving vaccinations. The confirmed rate of all serious side effects for the SARS-CoV-2 vaccines are about 1 in 500000 people inoculated which is far better than the death rate.
The mortality rate within 60 days of a vaccination visit was 4425 deaths per 100000 person-years.